Sections
Background
In 1994, ICNARC established the national clinical audit for adult critical care; the Case Mix Programme (CMP). Over 90% of adult, general critical care units in England, Wales and Northern Ireland now participate. Following rigorous data validation, all participating units receive regular, quarterly comparative reports for local performance management and quality improvement.
In line with many other national clinical audits, ICNARC makes results from the CMP public to provide a valuable insight into the quality of adult, general critical care both overall, and at the following levels:
- Strategic Health Authority
- Critical Care Network
- Trust or Health Board
- Hospital
- Individual critical care unit
Publication process
The Case Mix Programme (CMP) Annual Quality Report follows a clear publication process:
- All participating adult, general critical care units who have collected and submitted data from the period 1 April and 31 March of the reporting year are identified
- These units have been asked to agree to public disclosure of their results and have been given the option to consent (or not) to this disclosure
- To obtain consent, ICNARC has requested signatures from both the Clinical Director of each participating unit, as well as from the Chief Executive of the Trust
- If analysis reveals a participating unit with a potential outlier, ICNARC follows a clear 'Detection and management of outliers' policy (see appendix: Detection and management of outliers)
For the purposes of analysis, the database was originally locked on 9 August 2012. ICNARC have updated the 2011/12 report using additional data received after this date, up until the database was locked on 12 February 2013. Data received and/or validated after this date are not included.
93% of all eligible units (see appendix: Eligibility) are included in this report, compared with 90% of all eligible units in the 2010/11 Annual Quality Report.
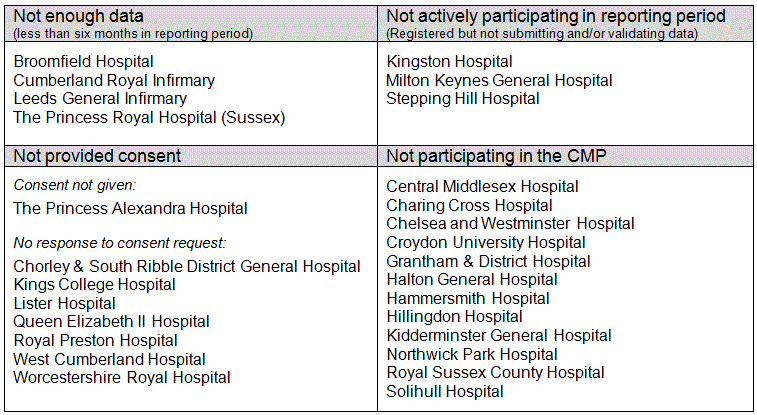
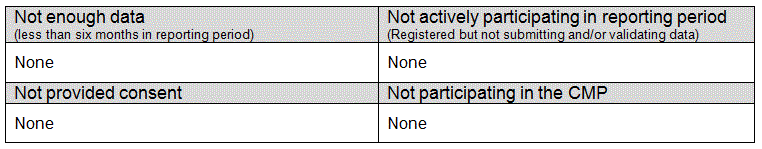
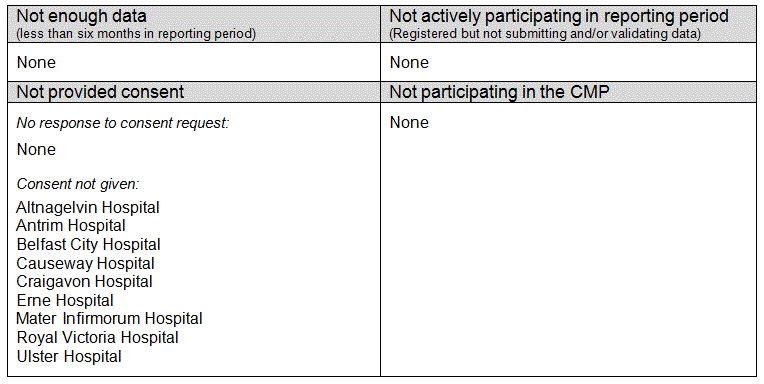
Critical care units eligible for, but not included in the 2011/12 Annual Quality Report - April update, are listed by country below.
England
Wales
Northern Ireland
Navigating this report
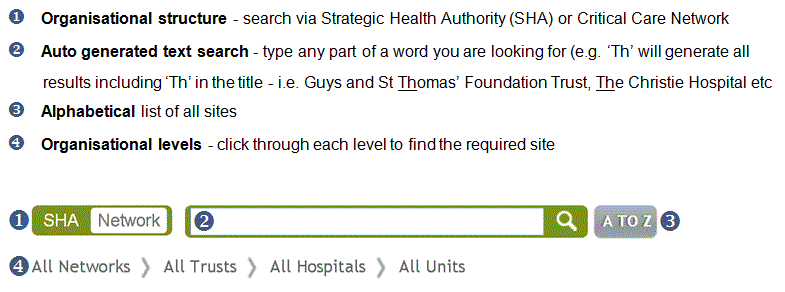
Results for all eligible sites can be found using various ways. See guidance and examples below to find out how to search for a required site and results.
Search for your unit or site via:
Once you have selected a site (including ‘all’), you can view results for each potential quality and participation indicator as follows:
- Click through each results tab
- View results graphically. For more information about the graphs used in this report, see appendix: Presentation of results
- View the numbers behind the graphs, as well as definitions for each Potential Quality Indicator in the accompanying text under each graph
Quality Indicators
The following potential Quality Indicators are reported in the Annual Quality Report:
Hospital mortality
- Hospital mortality is defined as death before ultimate discharge from acute hospital
- A mortality ratio is calculated by dividing the observed by the expected acute hospital mortality, with the expected estimated by a risk prediction model, a mortality ratio is one (1.0) when the observed and expected acute hospital mortality are equal
- The ICNARC model was developed and validated using Case Mix Programme (CMP) data and is regularly recalibrated to ensure accuracy
- The ICNARC risk prediction model describes the relationship between case mix factors (from the first 24 hours following admission to a critical care unit) and hospital mortality
- The ICNARC model estimates the expected number of acute hospital deaths for a given critical care unit based on the case mix of its admissions
Unit-acquired Methicillin-resistant Staphylococcus aureus (MRSA)
- Presence of MRSA in any sample taken for microbiological examination after 48 hours following admission to your unit – reported as the rate per 1000 patient days among all patients staying at least 48 hours and excluding those with admission MRSA or no samples taken.
- The comparator value is the overall rate of unit-acquired MRSA from all critical care units in the CMP for the time period of the report
Non-clinical transfers (out)
- Critical care unit survivors discharged for comparable critical care to a Level 3 bed in an adult ICU or ICU/HDU in another acute hospital – reported as the percentage of all critical care unit survivors
- The comparator value is the overall percentage of non-clinical transfers (out) from all critical care units in the CMP for the time period of the report
Unplanned readmissions within 48 hours
- Critical care unit survivors discharged to a location within the same hospital but subsequently readmitted to the critical care unit within 48 hours, and the subsequent admission classified as unplanned – reported as the percentage of all critical care unit survivors discharged to a location within the same hospital
- The comparator value is the overall percentage of unplanned readmissions within 48 hours in all critical care units in the CMP for the time period of the report
Out-of-hours discharges to the ward (not delayed)
- Critical care unit survivors discharged from a unit to a ward in the same hospital out-of-hours (between 22:00 and 06:59) who are not delayed i.e. not declared fully ready for discharge by 18:00 on that day – reported as the percentage of all unit survivors discharged to a ward in the same hospital
- The comparator value is the overall percentage of out-of-hours discharges to the ward (not delayed) in all critical care units in the CMP for the time period of the report
Other results included in the Annual Quality Report:
Active participation
- CMP participation is based on critical care units participating during the time period of the report - reported as the percentage of the possible NHS, adult general critical care unit(s) within the selection
- Available data are based on eligible (see appendix: Eligibility) units with data for each quarter – reported as the percentage of all participating units within the selection
Data completeness
- Data completeness is based on all admissions to the selection
- Indicates the level of completeness of data in all fields used to calculate each Potential Quality Indicator
Quality Indicators are presented in a funnel plot format (see appendix: Presentation of results)
Contact us
For questions or feedback on or about this Annual Quality Report, please contact ICNARC via email: cmp@icnarc.org